Abstract
Introduction: Post-transplant lymphoproliferative disorder (PTLD) is a rare and often aggressive disease in the setting of immunosuppression following solid organ transplant (SOT). Epstein-Barr virus (EBV) infection of B cells is responsible for about 50% of cases, either due to reactivation of the virus after transplantation or primary EBV infection. Although there is no approved therapy for patients (pts) with PTLD, guidelines include reduction of immunosuppression (RIS) as a part of initial treatment and may be sufficient for pts with early lesions. Rituximab, either as monotherapy or in combination with chemotherapy (CT), is used in addition to RIS as initial treatment. Pts with EBV + PTLD following SOT who fail rituximab plus CT have poor outcomes with limited treatment options. There are ongoing clinical studies investigating innovative therapies to address unmet needs in these pts. Published data on clinical outcomes of these pts remain limited and not well documented. We aimed to characterize the outcomes for pts diagnosed with EBV + PTLD following SOT who fail initial rituximab plus CT in a multi-national real-world setting.
Methods: We conducted a large multinational, multicenter, retrospective chart review study of pts with EBV + PTLD following allogeneic hematopoietic cell transplantation (HCT) or SOT who received rituximab or rituximab plus CT between January 2000‒December 2018 and were refractory (failed to achieve complete response [CR] or partial response [PR]) or relapsed at any point after such therapy. Treatment response was assessed either by clinical diagnosis, radiographic/imaging, biopsy/cytology, or a combination of such assessments. Data were collected from 29 centers across North America (United States and Canada) and the European Union. Study population was aligned to the ongoing investigational trial (Clinicaltrials.gov Identifier: NCT03394365). This analysis includes pts with EBV + PTLD following SOT who were refractory/relapsed to rituximab plus CT. The Kaplan-Meier method was utilized to estimate the overall survival (OS).
Results: A total of 86 pts with EBV + PTLD following SOT who failed rituximab plus CT were included in the analysis; 65 (75.6%) pts were refractory while 21 (24.4%) relapsed after initial response of CR or PR. Median age at PTLD diagnosis was 43 years (range 1‒78) and median time to PTLD onset from transplant was 1.7 years (range 0.1‒27.9). Median follow up time was 12.9 months from the date of PTLD diagnosis. PTLD histological subtypes were 66 (76.7%) monomorphic, 18 (20.9%) polymorphic, and 2 (2.3%) early lesions. The most common PTLD subtype was diffuse large B-cell lymphoma (DLBCL) (58, 67.4%).
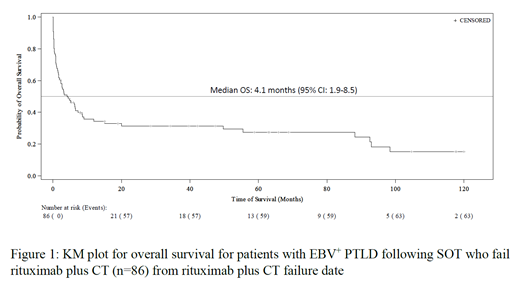
Of the 86 pts, 49 (57%) received CT following rituximab monotherapy while 37 (43%) pts received CT concurrently with rituximab. Overall, 63 (73.3%) pts died. PTLD-specific mortality was observed in 41 (65.1%) pts, treatment-related mortality in 10 (15.9%) pts, mortality due to organ rejection/failure in 2 (3.2%) pts, mortality due to other causes in 7 (11.1%) pts, and mortality due to unknown causes in 3 (4.8%) pts. Median OS was 15.5 months (95% confidence interval [CI]: 8.3‒22.9) from PTLD diagnosis, and was 4.1 months (95%CI: 1.9‒8.5) from the earliest date when pts became refractory or relapsed following rituximab plus CT.
Conclusions: The prognosis for pts with EBV + PTLD following SOT who fail rituximab plus CT remains poor, with an estimated median OS of about 4 months and a majority of pts dying from PTLD and related treatment. In this specific population, there remains a significant unmet medical need for effective and well-tolerated therapies.
Dharnidharka: Merck, Pfizer, Medronic, Cardinal Health: Current holder of stock options in a privately-held company; CareDx: Honoraria, Research Funding; Atara Bio, MedinCell: Consultancy. Thirumalai: Atara Biotherapeutics: Current Employment. Jaeger: Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Norvartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Zhao: Atara Biotherapeutics: Current Employment. Dierickx: Roche: Consultancy, Research Funding; Novartis: Consultancy; Sanofi: Consultancy; Sandoz: Consultancy; Takeda, Incyte, Atara: Consultancy. Xun: Atara Biotherapeutics: Current Employment. Sawas: Flat Iron Health: Current Employment; Roche: Current holder of stock options in a privately-held company; Seattle Genetics, Acrotech: Consultancy; Daiichi-Sankyo, Seattle Genetics, Gilead: Other: Travel, accommodation, expenses, Speakers Bureau; Affimed, Trillium: Research Funding. Sadetsky: Atara Biotherapeutics: Current Employment. Barlev: Atara Biotherapeutics: Current Employment. Zimmermann: Roche, Atara: Research Funding; Celgene, Roche, Atara, Jansen: Other: Travel accomodations. Trappe: Celgene: Other: Travel support; Roche: Other: Travel support; Atara Biotherapeutics: Consultancy, Other: Travel support, Research Funding; Jansen: Other: Travel support; GSK: Other: Travel support; AbbVie: Other: Travel support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal